IS THE QUANTITATIVE MEASUREMENT OF IMMATURE GRANULOCYTES ON SYSMEX XN-1000 HEMATOLOGY ANALYZER TRULY RELIABLE?
Introduction
Immature granulocytes are early-stage neutrophilic cells that mature in the bone marrow before being released into the bloodstream. The production of mature granulocytes is affected by outer or inner stimuli of the bone marrow.
An increased number of immature granulocytes (IGs) in the peripheral blood is an indicator of emergency granulopoiesis, a response mechanism of the bone marrow leading to releasing premature granulocytes to the bloodstream. A high IG count can be found in cases of infection, inflammation, and sepsis, but also diseases of bone marrow [1]. Although the white blood cell count continues to attract the most attention, it is least useful, and evidence suggest that emphasis should be shifted to other complete blood count (CBC) parameters, one of which is the IG fraction [2,3]. Immature granulocyte percentage is a useful marker to predict infection and its severity [4]
Elevated immature granulocyte count is associated with severe outcomes and can indicate infection, inflammation, or sepsis.
Clinical decision
The IG count can act as an indicator for sepsis and inflammatory conditions such as rheumatoid arthritis [5, 6, 7]. According to a study by Georgakopoulou et al., greater IG values were associated with severe and critical cases of SARS-CoV-2 infection [8]. Other infections, such as febrile urinary tract infections in children can use the IG count as an early detection marker [9]. In neonates, it is considered that immature granulocytes can be present in peripheral blood in smaller amounts, as a response to bone marrow’s high production of cells. Ianni et al. have even tried to establish a reference interval for such values for newborn population [10]. In some other immature to total neutrophil ratio (I/T ratio) of newborn population has been explored as an indicator of early onset neonatal sepsis [11]. Nigro et al. have also studied the newborn population, and their results and conclusions were similar, but referred only to the IG count [12]. Meanwhile, Wettin et al. have found that low counts of immature granulocytes may indicate neonatal infection [13]. One study of pregnant women has shown immature granulocytes to be an independent risk factor for predicting gestational diabetes mellitus, preterm delivery and macrosomia [14]. The diagnostic use of immature granulocytes is highly explored for different infections and inflammatory conditions, and the results are pointing out that the IG count is largely underutilized. It can also be combined with other inflammatory markers, such as CRP, to assess the severity of an inflammatory condition as it can be determined from routine laboratory test results and requires no additional intervention or cost [15, 16].
Automated hematology analyzers (HA) such as the Sysmex XN-1000 are widely used to perform the complete blood count and offer information on abnormal cells such as immature granulocytes, abnormal lymphocytes and blasts.
The IG count comprises metamyelocytes, myelocytes and promyelocytes. Blasts and band neutrophils are not considered immature granulocytes.
Manual microscopy is still the reference method for IG %, according to CLSI H20-A2 guidelines [17]. It’s time and labor intensive and demands significant expertise.
Also, a manual count is often imprecise, as it is composed of a relatively small number of cells. Additionally, it’s susceptible to human error and subjective judgement.
In contrast, the IG count given by the automated HA is available in only a few minutes, which is particularly important for laboratories with high throughput. Also, the results are consistent, standardized and more uniform.
Sysmex XN-1000 is a widely used hematology analyzer and immature granulocytes show great results as an indicator of inflammatory and infectious conditions. Patient samples used in our study comprised all samples with a measured value of IGs, resulting in a wide range of values 0.7 – 23.3 % IG. Taking in all the samples with an IG value ensured a variety of conditions causing immature cells appearing in the bloodstream. The differences in study results on the IG% threshold and protocols used to evaluate the IG concentration measurement encouraged us to analyze XN-1000 by the CLSI and ICHS guidelines [17, 18, 19].
All the inconsistencies regarding the IG % threshold and trustworthiness of the Sysmex HAs pose a problem with reporting assessment of the IG % parameter within the six-part differential. The aim of this study was to verify the quantitative IG count of the Sysmex XN-1000 HA and to assess its degree of association with the manual count with the goal of reducing blood film review rate without compromising clinical reliability.
Materials and Methods
The experimental study was performed during October and November 2024 at the Department of Medical Laboratory Diagnostics, University Hospital Sveti Duh, Zagreb, Croatia.
The verification protocol included analyzing Precision, Comparability, Limit of Blank (LoB) and Accuracy (comparability with the reference method) [20].
Precision, Comparability and Limit of Blank
A short precision study was conducted including repeatability and total precision. The repeatability study was performed using three patient samples in three different concentration ranges and commercial quality control samples SYSMEX XN CHECK™ (lot 4246, exp: 11/24) on three concentration levels, across 20 replicates. The total precision study was carried out utilizing commercial quality control samples SYSMEX XN CHECK™ (lot 4246, exp: 11/24) on three levels, repeated over a 30-day period.
Since the study on immature granuloc ytes was performed on two identical Sysmex XN-1000 analyzers, a short comparison study was performed to ensure data comparability was maintained. Forty patient samples were included in the comparison study.
Verification of the Limit of Blank (LoB) was achieved by measuring a blank sample (containing only water) across 20 replicates [21].
Manufacturers’ acceptance criteria for the Precision, Comparability and Limit of Blank were used. Coefficients of variation (CVs) were compared.
Accuracy
The accuracy of the Sysmex XN-1000 HA on IG concentration measurement was established by comparison to the reference method, a manual smear review. One hundred whole blood K₂EDTA samples were collected, broadly varying in the IG concentration range. The accuracy was verified through comparing the IG count by the Sysmex HA to the IG count obtained by the smear review. Blood smears were stained using the May Grünwald Giemsa (MGG) staining technique and examined on the Olympus BX53 microscope. For every blood sample, two smears were prepared, and a 200-cell manual differential was performed by two experienced medical laboratory professionals, according to CLSI H20-A2:2007 guidelines [17]. The number of promyelocytes, myelocytes and metamyelocytes counted by the manual smear review were added together, in order to enable comparison to the IG% count offered by the Sysmex HA.
Statistical analysis
The precision study results were expressed as imprecision for both repeatability and total precision and were reported as coefficient of variation [CV (%)]. An average bias was calculated using the Bland-Altman plot for both the comparison between the Sysmex HA, as well as the comparison between the automated and manual method.
Table 1: Results of the repeatability study for measurement of immature granulocytes on Sysmex XN-1000 hematology analyzer
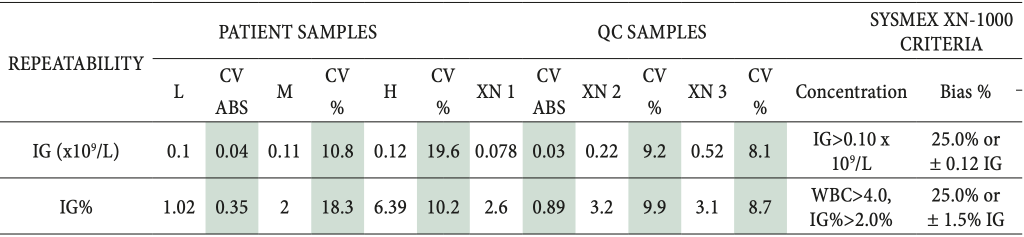
L, M, H – patient samples with low, medium and high concentration of immature granulocytes.
XN 1, 2, 3 – quality control samples for Sysmex XN-1000 (Level 1, Level 2 and Level 3).
Green color – acceptable results.
The latter was employed in assessing the accuracy of the Sysmex’s IG count. In addition, a rank correlation for both comparisons was performed. Statistical analysis was performed in MedCalc® v23.1.1 statistical software (MedCalc Software Ltd, Ostend, Belgium). All the results were evaluated in contrast to the manufacturer’s acceptance criteria. P-Value<0.05 was considered statistically significant.
Results
Precision
The repeatability results presented in Table 1 were within the acceptance criteria (25.0 % or 0.12 x 109/L IG) on all three concentration levels for both patient and quality control samples. The QC samples showed slightly lower CV (up to 9.9 %) rather than patient samples (which were up to 19.6 % for high IG concentrations). The total precision results from the quality control samples were also within the manufacturer-defined acceptance criteria (30 %) and are demonstrated in Table 2.
Comparability and LoB
Strong correlation (correlation coefficient 0.98, 95 % CI: 0.97 – 0.99, p-value<0.0001) that exceeds the established manufacturers criteria (correlation coefficient>0.40) was observed between the analyzers. The verification of the Limit of Blank also matched the criteria, with all results.
Accuracy
The accuracy results were calculated using IG count obtained by the manual smear review as the reference method. The Bland-Altman plot revealed a positive average bias of 1.2% IG (95% CI: 0.9 – 1.5, P<0.0001), which aligns with the manufacturer’s criteria (±1.5% IG). Rank correlation showed a satisfactory correlation coefficient of 0.90 (95% CI: 0.86 – 0.93, P<0.0001), which also corresponds to the defined criteria (correlation coefficient>0.80).
Discussion
The Sysmex XN-1000 HA meets the defined manufacturer’s IG count specifications. It shows great results within the precision study (comprising repeatability and total precision), comparability study between analyzers and LoB verification. To assess its accuracy, we compared the XN-1000’s IG counts with those obtained by manual microscopy, the established reference method, according to CLSI H20-A2:2007 guidelines. Our results indicate that Sysmex HA delivers reliable and reproducible IG counts within the six-part differential, suggesting that its automated IG measurements are sufficiently accurate for clinical use.
Table 2: Results of the total precision study for measurement of immature granulocytes on Sysmex XN-1000
hematology analyzer.
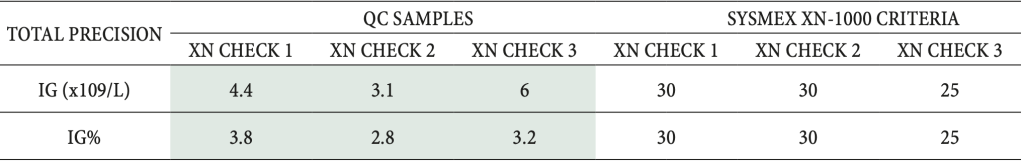
XN CHECK 1, 2, 3- quality control samples for Sysmex XN-1000 (Level 1, Level 2 and Level 3).
Green color- acceptable results.
An overestimation bias was observed on the automated IG count compared to manual microscopy, consistent with findings from previous studies also done on Sysmex’s hematology analyzers [22, 23, 24]. By evaluating a significantly larger cell population, the automated system detects lower IG levels that manual counts might miss due to their smaller sample size. Nevertheless, the positive bias remains within the manufacturer’s specifications.
Serrando Querol et al. compared the Beckmann Coulter DxH 900 to Sysmex XN20 analyzer and found that the DxH 900 shows better agreement for the IG count in all cases with manual microscopy, and also confirmed previously mentioned positive bias, especially for samples with IG % > 5 % [24]. Linko-Parvinen et al. have suggested utilizing different thresholds on Sysmex XN-1000 whether the IG % is reported or not. If the IG % is reported within the six-part differential, they suggest the threshold for a smear review to be 6 %, and if it is not reported they find the 3 % threshold more adequate [25]. When comparing the Sysmex XN-2000 to Horiba Yumizen H2500, the statistically significant difference of the IG % was explained by differences in methods used by the HAs. The Yumizen H2500 uses the impedance method in addition to the flow cytometry method to count immature granulocytes, in contrast to the XN-2000 using the fluorescent flow cytometry in all channels [26]. In another study, evaluation of Sysmex XN-9000 for detecting IGs in cases of myeloid neoplasms revealed some inconsistencies between XN-9000 and the manual count and ascribed it to inaccurate IG gating in the scattergram and morphologically abnormalities of immature cells [27]. Starks et al. analyzed autovalidation middleware data and raised the IG % threshold from 2 % to 5%, after finding it prevented 6 % of all samples from being autovalidated on Sysmex XN-9000 [28].
The different results obtained by the mentioned studies can be due to many factors. The specific patient group, e.g. patients with a myeloid neoplasm or children can make the results biased. Variations in protocols, including the use of only one slide in the study, or a smaller total cell count number in the smear review (standard 100-cell differential) deviate from established guidelines, and make the results less reliable. Furthermore, Sysmex HAs use the same method for counting immature granulocytes, but vary from other hematology analyzers, making the threshold harder to standardize. Laboratories that use autovalidation also struggle to assess the accuracy of the flag, as they cannot afford to overlook any cases by autovalidation protocol.
The manual smear is prone to different mistakes, such as losses due to the fact that IGs are larger cells located at the feathered ends of the smear and smaller total cell count, besides the already mentioned reasons.
The reliability of the automated IG count supports its potential to alleviate a heavy workflow by reducing the smear review rate. The analyzer’s reproducibility and consistency minimize the subjectivity and excess labor associated with a manual smear review. Furthermore, the HA generates the IG count within minutes as part of the six-part differential, improving turnaround time and workflow efficiency.
Emergency medicine relies on rapid turnaround, and the Sysmex XN-1000 provides automated, reliable IG counts within every CBC, enabling results within minutes for timely clinical decisions.
However, the XN-1000 does not morphologically differentiate between different WBC precursor cells (promyelocytes, myelocytes and metamyelocytes), which could pose a problem with specific patient groups and complex hematology cases. The analyzer’s limitations should be considered when setting the IG % threshold for warranting a smear review.
This study evaluates XN-1000’s ability to measure immature granulocytes ranging from very low concentrations not reported by the flagging system to very high ones appearing in cases of severe bone marrow response. The analyzers used in our laboratory are comparable and reliably provide IG concentration results.
In summary, the XN-1000 offers trustworthy results and is an efficient alternative to a manual smear review regarding the IG count. To ensure accurate diagnoses, one must take into consideration the automated IG count in context of each clinical case. Sysmex XN-1000 provides reliable quantitative IG measurement results, effectively complementing manual microscopy. Automated IG reporting could be incorporated as a part of the CBC, thus significantly improving turnaround time in severe cases where every second to diagnosis count.
References
- McKnezie SB, Landis-Piwowar K, Williams JL. Clinical Laboratory Hematology. 4ed 2020
- Farkas JD. The complete blood count to diagnose septic shock. J Thorac Dis. 2020 Feb;12(Suppl 1):S16-S21. doi: 10.21037/jtd.2019.12.63.
- Mare TA, Treacher DF, Shankar-Hari M, Beale R, Lewis SM, Chambers DJ, Brown KA. The diagnostic and prognostic significance of monitoring blood levels of immature neutrophils in patients with systemic inflammation. Crit Care. 2015 Feb 25;19(1):57. doi: 10.1186/s13054-015-0778-z.
- van der Geest PJ, Mohseni M, Brouwer R, van der Hoven B, Steyerberg EW, Groeneveld AB. Immature granulocytes predict microbial infection and its adverse sequelae in the intensive care unit. J Crit Care. 2014 Aug;29(4):523-7. doi: 10.1016/j.jcrc.2014.03.033.
- Bhansaly P, Mehta S, Sharma N, Gupta E, Mehta S, Gupta S. Evaluation of Immature Granulocyte Count as the Earliest Biomarker for Sepsis. Indian J Crit Care Med. 2022 Feb;26(2):216-223. doi: 10.5005/jp-journals-10071-23920.
- Nierhaus A, Klatte S, Linssen J, Eismann NM, Wichmann D, Hedke J, Braune SA, Kluge S. Revisiting the white blood cell count: immature granulocytes count as a diagnostic marker to discriminate between SIRS and sepsis–a prospective, observational study. BMC Immunol. 2013 Feb 12;14:8. doi:10.1186/1471-2172-14-8.
- Özcan E, Gülten S. A new inflammation marker in rheumatoid arthritis: immature granulocyte. Kırıkkale Uni Med J. April 2023;25(1):56-63. doi:10.24938/kutfd.1143318
- Georgakopoulou VE, Makrodimitri S, Triantafyllou M, Samara S, Voutsinas PM, Anastasopoulou A et al. Immature granulocytes: Innovative biomarker for SARSCoV2 infection. Mol Med Rep. 2022 Jul;26(1):217. doi: 10.3892/ mmr.2022.12733. Epub 2022 May 13.
- Cetin N, Kocaturk E, Tufan AK, Kiraz ZK, Alatas O. Diagnostic Values of Immature Granulocytes Detected by the Sysmex XN 9000 Hematology Analyzer in Children with Urinary Tract Infections. Saudi J Kidney Dis Transpl. 2023 Dec 1;34(Suppl 1):S133-S141. doi: 10.4103/sjkdt.sjkdt_33_22. Epub 2024 Jul 3.
- Ianni B, McDaniel H, Savilo E, Wade C, Micetic B, Johnson S, Gerkin R. Defining Normal Healthy Term Newborn Automated Hematologic Reference Intervals at 24 Hours of Life. Arch Pathol Lab Med. 2021 Jan 1;145(1):66-74. doi: 10.5858/arpa.2019-0444-OA.
- Saboohi E, Saeed F, Khan RN, Khan MA. Immature to total neutrophil ratio as an early indicator of early neonatal sepsis. Pak J Med Sci. 2019 Jan- Feb;35(1):241-246. doi: 10.12669/pjms.35.1.99.
- Nigro KG, O’Riordan M, Molloy EJ, Walsh MC, Sandhaus LM. Performance of an automated immature granulocyte count as a predictor of neonatal sepsis. Am J Clin Pathol. 2005 Apr;123(4):618-24. doi: 10.1309/73H7-K7UB- W816-PBJJ.
- Wettin N, Drogies T, Kühnapfel A, Isermann B, Thome UH. Automated Complete Blood Cell Count Using Sysmex XN-9000® in the Diagnosis of Newborn Infection. J Clin Med. 2022 Sep 20;11(19):5507. doi: 10.3390/ jcm11195507.
- Wang W, Meng X, Sun Y, Yin B, Ding L, Zhang L, Ma M, Zhu B, Shen Y. Immature granulocytes are closely associated with the development of maternal gestational diabetes mellitus and adverse pregnancy outcomes. J Biol Regul Homeost Agents. 2024, 38(6): 4717-4724 https://doi.org/10.23812/j. biol.regul.homeost.agents.20243806.376
- Incir S, Kant Calti H, Palaoglu KE. The role of immature granulocytes and inflammatory hemogram indices in the inflammation. Int J Med Biochem. 2020;3(3):125-130
- Jeon K, Lee N, Jeong S, Park MJ, Song W. Immature granulocyte percentage for prediction of sepsis in severe burn patients: a machine leaning-based approach. BMC Infect Dis. 2021 Dec 16;21(1):1258. doi: 10.1186/s12879- 021-06971-2.
- CLSI H20-A2:2007 Reference Leukocyte (WBC) Differential Count (Proportional) and Evaluation of Instrumental Method, 2nd Edition.
- International Council for Standardization in Haematology, Writing Group; Briggs C, Culp N, Davis B, d’Onofrio G, Zini G, Machin SJ; International Council for Standardization of Haematology. ICSH guidelines for the evaluation of blood cell analysers including those used for differential leucocyte and reticulocyte counting. Int J Lab Hematol. 2014 Dec;36(6):613-27. doi: 10.1111/ijlh.12201.
- CLSI H26-A2:2010 Validation, Verification, and Quality Assurance of Automated Hematology Analyzers; Approved Standard—Second Edition
- Vis JY, Huisman A. Verification and quality control of routine hematology analyzers. Int J Lab Hematol. 2016 May;38 Suppl 1:100-9. doi: 10.1111/ ijlh.12503.
- Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008 Aug;29 Suppl 1(Suppl 1):S49-52. PMID: 18852857; PMCID: PMC2556583.
- Eilertsen H, Hagve TA. Do the flags related to immature granulocytes reported by the Sysmex XE-5000 warrant a microscopic slide review? Am J Clin Pathol. 2014 Oct;142(4):553-60. doi: 10.1309/AJCP4V4EXYFFOELL.
- Cherian S, Levin G, Lo WY, Mauck M, Kuhn D, Lee C, Wood BL. Evaluation of an 8-color flow cytometric reference method for white blood cell differential enumeration. Cytometry B Clin Cytom. 2010 Sep;78(5):319-28. doi: 10.1002/ cyto.b.20529.
- Serrando Querol M, Nieto-Moragas J, Marull Arnall A, Figueras MD, Jiménez-Romero O. Evaluation of the New Beckmann Coulter Analyzer DxH 900 Compared to Sysmex XN20: Analytical Performance and Flagging Efficiency. Diagnostics (Basel). 2021 Sep 24;11(10):1756. doi: 10.3390/ diagnostics11101756.
- Linko-Parvinen AM, Kurvinen K, Tienhaara A. Accuracy of Sysmex XN immature granulocyte percentage compared to manual microscopy. J Lab Precis Med. 2021;6:27
- Małecka M, Ciepiela O. A comparison of Sysmex-XN 2000 and Yumizen H2500 automated hematology analyzers. Pract Lab Med. 2020 Oct 29;22:e00186. doi: 10.1016/j.plabm.2020.e00186.
- Lu Q, Li Y, Li T, Hou T, Zhao Y, Feng S, Yang X, Zhu M, Shen Y. Evaluation of immature granulocyte parameters in myeloid neoplasms assayed by Sysmex XN hematology analyzer. J Hematop. 2022 Mar;15(1):1-6. doi: 10.1007/ s12308-022-00484-w. Epub 2022 Feb 8.
- Starks RD, Merrill AE, Davis SR, Voss DR, Goldsmith PJ, Brown BS, Kulhavy J, Krasowski MD. Use of Middleware Data to Dissect and Optimize Hematology Autoverification. J Pathol Inform. 2021 Apr 7;12:19. doi: 10.4103/jpi. jpi_89_20.

Published under the Creative Commons Attribution 4.0 International License
