VASOPRESSORS IN TRAUMA PATIENTS: A REVIEW OF INDICATIONS, BENEFITS, AND CONTROVERSIES
Introduction
Trauma-induced hypotension is a leading cause of morbidity and mortality in emergency and critical care settings (1-4). While fluid resuscitation and hemorrhage control are cornerstones of trauma management, the use of vasopressors remains a controversial topic (5). Vasopressors, which include agents such as norepinephrine, epinephrine, vasopressin, dopamine, and phenylephrine, are frequently used in critically ill patients to support blood pressure and organ perfusion. However, their use in trauma patients, especially those in hypovolemic shock, has raised concerns due to their potential to exacerbate tissue ischemia and complicate management.
The debate surrounding vasopressor use in trauma arises from the delicate balance between maintaining perfusion to vital organs and the possible adverse effects of these medications. Vasopressors work by constricting peripheral blood vessels, thereby increasing systemic vascular resistance and improving blood pressure. However, they may reduce blood flow to the microcirculation, impairing oxygen and nutrient delivery to tissues, particularly in already compromised areas. Additionally, excessive vasoconstriction can increase myocardial oxygen demand and contribute to complications such as arrhythmias, organ dysfunction, and even death.
In trauma patients, where rapid resuscitation and hemorrhage control are paramount, the timing and judicious use of vasopressors are critical. While vasopressors can be life-saving, they are not without significant risks, and their role in trauma care is still evolving. This article will explore the pathophysiology of trauma and shock, the rationale for and against vasopressor use in trauma patients, and the current clinical evidence that guides their use in trauma care.
Pathophysiology of Traumatic Shock
Trauma-induced shock is predominantly classified as hypovolemic shock, resulting from significant blood loss, fluid shifts, or a combination of both. This loss of effective circulating blood volume leads to a critical reduction in tissue perfusion and oxygenation. In response to trauma, the body initiates a two-phase physiological response to counteract the effects of shock and preserve perfusion to vital organs (6).
In the early stages of trauma-induced shock, the sympathetic nervous system (SNS) is activated as the primary compensatory mechanism to preserve perfusion to vital organs (7). This activation results in the release of catecholamines, including norepinephrine, epinephrine, and dopamine, which exert their effects on adrenergic receptors throughout the cardiovascular system and peripheral tissues to maintain hemodynamic stability.
Hemorrhage precipitates a rapid and pronounced surge in catecholamine levels, particularly epinephrine and norepinephrine, which can increase by 10 to 40 times their normal baseline concentrations (8). These catecholamines act on β-adrenergic receptors to increase heart rate (chronotropy) and myocardial contractility (inotropy), thereby enhancing cardiac output. Simultaneously, they engage α-adrenergic receptors to induce peripheral vasoconstriction, redirecting blood flow to critical organs such as the brain and heart while maintaining systemic vascular resistance and blood pressure. This neurohumoral response ensures the temporary stabilization of vital physiological functions during the acute phase of hypovolemic shock, buying crucial time for interventions to address the underlying cause of blood loss.
One of the primary responses to trauma is tachycardia, where the heart rate increases to compensate for the decreased stroke volume and circulating blood volume. This helps to maintain cardiac output and support organ perfusion. At the same time, the SNS triggers widespread vasoconstriction through the release of norepinephrine and epinephrine, which act on alpha-adrenergic receptors in vascular smooth muscle. This causes an increase in systemic vascular resistance (SVR) and elevates blood pressure, helping to direct blood flow to critical organs such as the brain, heart, and kidneys. In parallel, blood is preferentially shunted away from less vital organs, including the gastrointestinal tract, skin, and muscles, in an effort to preserve perfusion to the organs most vital for survival.
Additionally, the activation of the renin-angiotensin-aldosterone system (RAAS) plays a crucial role in the body’s compensatory response. The drop in blood pressure triggered by the loss of blood volume stimulates the kidneys to release renin, which leads to the production of angiotensin II. Angiotensin II acts as a potent vasoconstrictor, further increasing SVR and supporting blood pressure. It also stimulates the release of aldosterone, which promotes sodium and water retention, attempting to restore circulating volume and prevent further hypotension. Together, these mechanisms work to mitigate the effects of hypovolemic shock and sustain critical physiological functions (fig. 1).
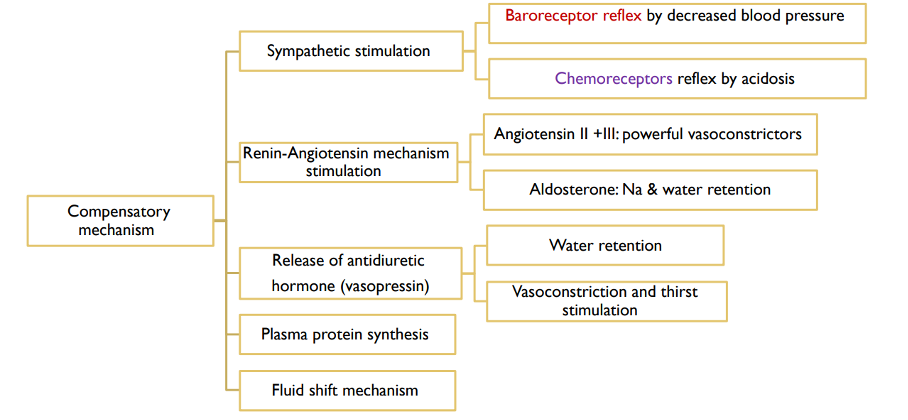
Figure 1. Compensatory mechanisms of haemorrhagic shock
While these compensatory mechanisms initially help maintain blood flow to vital organs, their effectiveness decreases over time, especially in severe trauma. Prolonged activation of the sympathetic nervous system (SNS) leads to a downregulation of catecholamine receptors, particularly beta-adrenergic receptors. This reduces receptor sensitivity, making the body less responsive to catecholamines. Despite continued release of these hormones, it becomes increasingly difficult to maintain blood pressure and tissue perfusion, signaling the progression to the second phase of shock.
In addition, prolonged vasoconstriction can cause end-organ ischemia, limiting oxygen delivery to tissues. Due to end-organ ischemia and impaired oxygen delivery, anaerobic metabolism ensues leading to metabolic acidosis (9). The acidosis further worsens the situation by inactivating and reducing the number of vasopressor receptors, weakening the body’s ability to stabilize circulation. These changes highlight the importance of timely treatment to prevent shock from advancing and causing permanent organ damage.
Trauma-induced hypotension is a major cause of morbidity and mortality in emergency and critical care, with vasopressors used to support blood pressure but remaining controversial in trauma management.
As the trauma progresses, the body enters a depressive phase, marked by the failure of compensatory mechanisms and the onset of organ dysfunction. The ongoing hemorrhage, coupled with the inflammatory response to trauma, depletes the body’s ability to sustain sympathetic activation. This results in the loss of vasoconstriction and a decline in blood pressure, further compromising perfusion to tissues and organs. Endothelial dysfunction, triggered by inflammatory mediators, leads to microvascular injury, causing vasodilation and increased capillary permeability (10). This further exacerbates the loss of blood volume and impairs the ability of the microcirculation to deliver oxygen and nutrients to tissues, worsening tissue hypoxia and metabolic acidosis. Metabolic acidosis results from anaerobic metabolism, which occurs when oxygen supply to tissues is insufficient. The accumulation of lactic acid contributes to a decrease in pH, further impairing cellular function and exacerbating shock. Additionally, the inflammatory response, along with endothelial damage, contributes to coagulopathy, which impairs the blood’s ability to clot and prolongs hemorrhage. The combination of prolonged blood loss and coagulopathy worsens the hypovolemia, increasing the severity of shock and leading to multi-organ dysfunction. In this depressive phase, the body’s compensatory mechanisms are overwhelmed, and vital organ perfusion may deteriorate despite ongoing efforts to restore blood volume and pressure. Multi-organ dysfunction becomes more likely, and survival becomes increasingly dependent on effective and timely interventions. These interventions include fluid resuscitation to restore blood volume, hemorrhage control to stop the source of bleeding, and in some cases the careful use of vasopressors such as norepinephrine or vassopresin to maintain blood pressure and support organ perfusion. Vasopressors can help to stabilize hemodynamics and improve tissue perfusion when the body’s own compensatory mechanisms are no longer sufficient support perfusion and stabilize blood pressure.
Understanding the two-phase response in trauma-induced shock – beginning with sympathetic activation and catecholamine release, followed by receptor downregulation and failure of compensatory mechanisms – is critical for guiding therapeutic interventions. The proper management of trauma-induced shock requires timely interventions to address both the early compensatory phase and the later depressive phase, with the goal of improving tissue perfusion and preventing organ failure.
Shock-Induced Endotheliopathy
As already emphasized, a significant factor contributing to poor outcomes in trauma patients is endothelial injury and dysfunction. In hemorrhagic shock, the endothelium is subjected to inflammatory mediators, shear stress, and ischemia-reperfusion injury, all of which contribute to a condition known as shock-induced endotheliopathy (SHINE) (11,12). SHINE is a critical factor in the pathophysiology of post-traumatic hemorrhagic shock and plays a key role in the progression of shock-related complications. The endothelium is crucial for maintaining vascular homeostasis, with functions that include preserving vascular patency, regulating fluid permeability, and controlling vasomotor tone. Furthermore, the endothelium contributes to natural anticoagulation and the maintenance of vascular integrity through its glycocalyx, a protective layer made of glycoproteins and proteoglycans that houses important anticoagulant molecules like heparinoids and antithrombin.
In the context of SHINE, endothelial dysfunction occurs as a result of glycocalyx degradation, increased vascular permeability, and impaired anticoagulant properties. This dysfunction leads to microvascular thrombosis, edema, and tissue hypoxia. The damage to the glycocalyx is particularly harmful as it disrupts the normal endothelial barrier function, increasing the likelihood of clot formation and exacerbating inflammation. These changes contribute to a vicious cycle of further endothelial damage, impaired circulation, and compromised organ function, which worsens the overall severity of hemorrhagic shock.
The cumulative effects of endothelial dysfunction worsen the progression of shock, contributing to multi-organ failure and poor clinical outcomes. Addressing endotheliopathy is crucial for improving survival and recovery in hemorrhagic shock. Emerging therapies aim to preserve the glycocalyx and restore endothelial function. These strategies include fluid resuscitation with balanced crystalloids, antioxidants, and glycoprotein stabilizers, which help protect the endothelium from further damage. These approaches enhance the natural protective and anticoagulant properties of the endothelium, representing promising interventions to mitigate shock-induced endotheliopathy (SHINE) and improve clinical outcomes.
Indications for Vasopressors in Trauma (Why Not):
As we mention before, the use of vasopressors in trauma-induced hypotension, particularly in cases of hypovolemic shock, has long been a subject of debate in critical care. While vasopressors are indispensable in certain clinical scenarios such as septic or cardiogenic shock, their application in trauma presents unique challenges. The underlying pathophysiology of trauma-induced shock – characterized primarily by significant blood loss and hypovolemia – raises critical concerns about the efficacy and safety of vasopressors. Literature consistently emphasizes the need for caution regarding the routine use of vasopressors in trauma, highlighting the significant risks they pose to patients already in a fragile physiological condition (7,13,14).
Trauma-induced shock involves an initial compensatory phase with sympathetic activation, followed by a depressive phase with receptor downregulation and organ dysfunction. Timely interventions, like fluid resuscitation and vasopressors, are key to improving outcomes.
Vasopressors act by increasing systemic vascular resistance through vasoconstriction, which can lead to reduced perfusion in the microcirculation. In trauma-induced hypovolemia, the primary issue is the lack of circulating blood volume, not vascular tone (15). By further constricting blood vessels, vasopressors can worsen tissue ischemia and hypoxia, particularly in already compromised areas. This effect increases the risk of organ dysfunction and delayed recovery, as oxygen and nutrient delivery to tissues becomes insufficient.
In addition to their impact on tissue perfusion, vasopressors can aggravate the endothelial dysfunction inherent to trauma-induced shock. Vasopressors can intensify endothelial dysfunction by increasing shear stress, increased vascular permeability, and impaired anticoagulant properties of the endothelium (16). This worsens microvascular injury and contributes to the inflammatory cascade, leading to further complications such as edema, coagulopathy, and impaired oxygenation.
Another significant concern is the effect of vasopressors on myocardial oxygen demand. Vasopressors such as norepinephrine and epinephrine stimulate alpha- and beta-adrenergic receptors, increasing vascular resistance and heart rate. While this temporarily raises blood pressure, it also significantly increases myocardial oxygen demand. In the context of trauma, where the heart is already compensating for reduced preload and systemic hypoperfusion, this additional workload can precipitate cardiac dysfunction, arrhythmias, and myocardial ischemia.
A critical concern with the use of vasopressors in trauma management is their potential to mask the underlying hypovolemia (13,17). By artificially augmenting systemic arterial pressure, vasopressors can create a deceptive appearance of hemodynamic stability. This apparent normalization of blood pressure risks delaying definitive interventions, such as aggressive fluid resuscitation and rapid hemorrhage control, which are essential for correcting the primary cause of hypotension. Such delays may exacerbate the progression of shock and significantly compromise patient outcomes, as effective trauma care hinges on timely volume restoration and hemorrhage cessation to stabilize perfusion and prevent multi-organ failure.
In severe trauma, the prolonged release of endogenous catecholamines can lead to desensitization of adrenergic receptors (18). This downregulation diminishes the efficacy of both endogenous and exogenous vasopressors. As a result, reliance on these medications during the later stages of shock may produce diminishing returns, failing to effectively stabilize hemodynamics and potentially introducing additional risks, including worsened tissue perfusion.
Some study emphasizes that early vasopressor infusion in trauma patients increased the mortality rate, regardless of trauma severity (19,20). The underlying causes included low arterial pressure, higher fluid requirements, and elevated serum creatinine levels. These findings highlight the risks associated with the premature use of vasopressors in trauma patients, particularly when volume resuscitation and hemorrhage control have not been adequately addressed.
In conclusion, it is important to emphasize that the literature provides limited support for the routine use of vasopressors in trauma-induced hypovolemic shock. Current guidelines prioritize fluid resuscitation, blood product administration, and hemorrhage control as first-line therapies. Vasopressors are typically reserved for refractory cases where hypotension persists despite these interventions, and even then, their use must be carefully evaluated against the potential risks and complications. Use of vasopressors is not recommended according to the Advanced Trauma Life Support management principles (21).
Indications for Vasopressors in Trauma (Why Yes):
Despite studies linking vasopressor use to increased mortality in trauma patients (19,20), there are compelling reasons why vasopressors are increasingly being discussed and utilized in modern trauma management. One of the key factors contributing to this shift is the growing recognition of the complexities of trauma-induced shock, particularly hemorrhagic shock, and the limitations of traditional fluid resuscitation. Hemorrhagic shock, in particular, can be difficult to manage with volume resuscitation alone, especially in cases where blood loss is substantial, and organ perfusion cannot be adequately restored.
While early vasopressor use has been associated with higher mortality due to their potential to mask the underlying cause of hypovolemia and worsen ischemia, more recent literature suggests that in certain situations, low-dose vasopressors may be beneficial, particularly when initial fluid resuscitation fails to maintain adequate blood pressure and organ perfusion (7,22). A retrospective study highlighted that, in some cases, low-dose norepinephrine helped stabilize trauma patients and prevent progression to refractory shock, where conventional treatments may have failed (23). This evolving perspective reflects an increasing understanding that the management of trauma-induced shock is multifaceted and not solely reliant on volume resuscitation.
Moreover, the second phase of hemorrhagic shock, after bleeding has been controlled, can lead to a sepsis-like response triggered by ischemia/reperfusion (I/R) injury, which includes oxidative stress, and the systemic release of cytokines. These processes can significantly impair vascular tone and further compromise organ perfusion. Additionally, the administration of analgesic and sedative medications, which are essential for managing pain and agitation in hemorrhagic shock patients, can further compromise the vasoconstrictor response, exacerbating the effects of shock. These factors contribute to persistent hypotension and inadequate tissue perfusion, underlining the need for vasopressors to stabilize blood pressure, restore tissue perfusion, and prevent further complications in the critical phase of recovery (24).
Low-dose vasopressors, such as norepinephrine, are increasingly being used as adjuncts to volume resuscitation to stabilize blood pressure, improve tissue perfusion, and prevent multi-organ failure. These agents are typically reserved for cases where hypotension persists despite adequate fluid replacement and hemorrhage control. Research has demonstrated that, when carefully titrated, vasopressors can be a potentially lifesaving option in trauma care. A study by Zhang et al. (25) found that early use of norepinephrine improved survival rates in patients with traumatic hemorrhagic shock by helping to maintain adequate perfusion during the critical early stages of recovery.
Furthermore, the use of vasopressors in trauma care reflects an evolving understanding of the need for individualized treatment. While there are significant risks, such as exacerbating ischemia or delaying the recognition of hemorrhage, the careful titration of vasopressors offers a nuanced approach that can balance the need for blood pressure support with the potential for harm. A study by Gupta et al. (7) suggested that vasopressors may be particularly beneficial in trauma patients who experience ongoing hypotension despite sufficient fluid resuscitation, helping to prevent the transition to irreversible shock. This growing trend highlights the limitations of relying solely on fluids and blood products in severe traumatic shock. Vasopressors now play a key role in managing complex, life-threatening conditions, where simply addressing volume loss through resuscitation may not be sufficient.
This shift toward considering vasopressors as part of a broader, more personalized treatment strategy is also reflected in recent clinical guidelines, which recommend their use for cases of shock unresponsive to volume resuscitation (26). In conclusion, while the role of vasopressors in trauma management continues to evolve, their careful use in selected patients can offer significant benefits by maintaining vital organ perfusion and preventing further deterioration. As our understanding of trauma-induced shock deepens, the integration of vasopressors into treatment strategies will likely become more refined, ultimately improving patient outcomes during this critical phase of care.
More question than answer: which, when, and how much vasopressor?
The choice of vasopressor in trauma also remains a subject of ongoing debate. While current guidelines recommend norepinephrine as the first-line vasopressor for managing shock due to its well-established efficacy in improving blood pressure and organ perfusion, there is increasing interest in the potential role of vasopressin. Vasopressin, a potent vasoconstrictor with a different mechanism of action, has gained attention for its ability to enhance vascular tone without the negative effects on cardiac output seen with norepinephrine. Some studies suggest that vasopressin may be beneficial in certain traumatic shock cases.
Vasopressors should be used with caution in trauma-induced hypovolemic shock as they can worsen tissue ischemia, endothelial dysfunction, and delay necessary interventions like fluid resuscitation and hemorrhage control.
One of the most significant advantages of vasopressin in trauma patients is its ability to retain its pressor effects during severe acidosis and hypoxemia, conditions that often accompany traumatic shock (27). Unlike norepinephrine, whose vasoconstrictive properties may be diminished under these circumstances, vasopressin continues to function effectively in these critical conditions. This makes vasopressin particularly useful in refractory circulatory shock, where conventional vasopressors like norepinephrine may fail to restore adequate blood pressure and organ perfusion. Another notable benefit of vasopressin is its ability to inhibit nitric oxide (NO) synthesis, which plays a crucial role in maintaining vascular tone. In shock states, excessive NO production can exacerbate vasodilation, worsening hypotension and compromising tissue perfusion. By inhibiting NO synthesis, vasopressin counteracts the vasodilatory effects of NO, thereby helping to stabilize vascular tone and blood pressure (28). This action is particularly advantageous in trauma patients where excessive vasodilation can be a significant contributor to shock.
Additionally, vasopressin improves renal perfusion, which is essential in preventing acute kidney injury, a common complication in patients with severe trauma and shock. Vasopressin achieves this by causing vasodilation of the efferent renal arterioles, which contrasts with the vasoconstrictive properties of catecholamines such as norepinephrine. This mechanism helps maintain renal blood flow and glomerular filtration, reducing the risk of renal failure, which is often seen in critically ill patients requiring intensive resuscitation (29).
In contrast to norepinephrine, which can cause significant pulmonary vasoconstriction, vasopressin has a milder effect on the lungs (30). In fact, vasopressin can promote pulmonary vasodilation, which is particularly beneficial in trauma patients with respiratory compromise. This effect reduces the workload on the heart and improves oxygenation without exacerbating pulmonary hypertension, a common complication associated with high-dose catecholamine therapy.
Vasopressin also offers a significant advantage in terms of cardiac safety. High doses of norepinephrine are often associated with an increased incidence of arrhythmias, which can worsen outcomes in trauma patients with pre-existing cardiac instability. Vasopressin, on the other hand, has been shown to cause fewer arrhythmias compared to norepinephrine, making it a safer option for patients at risk for cardiac complications. This is particularly important in patients with traumatic injuries that may already stress the cardiovascular system, as minimizing arrhythmias can improve overall prognosis.
One of the unique features of vasopressin is its action as an indirect vasoconstrictor. It enhances the sensitivity of smooth muscle cells to circulating catecholamines, such as norepinephrine, which improves the efficacy of these agents at lower doses (31). This mechanism can reduce the need for high-dose norepinephrine or other catecholamines, minimizing the risk of adverse effects such as arrhythmias or excessive vasoconstriction, which can compromise organ perfusion.
Vasopressors can be useful in trauma management, particularly when fluid resuscitation alone fails to restore blood pressure and organ perfusion, helping to stabilize the patient and prevent further complications.
Moreover, vasopressin has immunomodulatory effects that can help mitigate the inflammatory response often triggered by trauma and shock (32). The inflammatory response in trauma patients can lead to widespread tissue damage, organ failure, and sepsis. By modulating the immune system, vasopressin may help reduce these harmful effects, promoting better recovery and reducing the incidence of secondary complications like infection.
In conclusion, vasopressin offers several distinct advantages over norepinephrine in the management of trauma-induced shock. Its ability to function in hypoxic and acidotic conditions, its effects on renal and splanchnic perfusion, and its relatively lower risk of arrhythmias make it a valuable adjunct to traditional vasopressors. While it should not replace norepinephrine as the first-line therapy in all cases, vasopressin has become an increasingly important tool in the management of refractory shock, offering a more targeted approach to treatment. As our understanding of trauma and shock physiology continues to evolve, vasopressin is likely to play an even greater role in modern trauma care, providing a safer and more effective option for critically ill patients.
Conclusion
The management of trauma-induced shock remains complex, with ongoing debates surrounding the use of vasopressors. Norepinephrine has long been the first-line treatment for shock, but increasing interest in vasopressin reflects a growing recognition of its potential benefits, particularly in severe, refractory cases. Vasopressin’s ability to maintain vascular tone in hypoxic and acidotic environments, its positive effects on renal and splanchnic perfusion, and its lower risk of arrhythmias offer several advantages over norepinephrine. However, its use must be carefully evaluated, as improper or premature administration of vasopressors can worsen ischemia and delay vital interventions. Ultimately, trauma care requires a personalized approach, and vasopressors should be employed based on the individual patient’s response to initial interventions, with fluid resuscitation and hemorrhage control as the primary strategies. As our understanding of trauma and shock physiology advances, vasopressin may play an increasingly pivotal role in optimizing outcomes for critically ill trauma patients.
Literature:
- Maheshwari K, Nathanson BH, Munson SH, Khangulov V, Stevens M, Badani H, et al. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 2018 Jun;44(6):857-67. doi: 10.1007/s00134-018-5218-5.
- Khanna AK, Maheshwari K, Mao G, Liu L, Perez-Protto SE, Chodavarapu P, Schacham YN, Sessler DI. Association Between Mean Arterial Pressure and Acute Kidney Injury and a Composite of Myocardial Injury and Mortality in Postoperative Critically Ill Patients: A Retrospective Cohort Analysis. Crit Care Med. 2019 Jul;47(7):910-917. doi: 10.1097/CCM.0000000000003763..
- Lipsky AM, Gausche-Hill M, Henneman PL, Loffredo AJ, Eckhardt PB, Cryer HG, et al. Prehospital hypotension is a predictor of the need for an emergent, therapeutic operation in trauma patients with normal systolic blood pressure in the emergency department. J Trauma. 2006 Nov;61(5):1228-33. doi: 10.1097/01.ta.0000196694.52615.84.
- Codner P, Obaid A, Porral D, Lush S, Cinat M. Is field hypotension a reliable indicator of significant injury in trauma patients who are normotensive on arrival to the emergency department? Am Surg. 2005 Sep;71(9):768-71.
- Hooper N, Armstrong TJ. Hemorrhagic Shock. 2022 Sep 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
- Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol. 1991 Feb;260(2Pt2):H305-18. doi: 10.1152/ajpheart.1991.260.2.H305.
- Gupta B, Garg N, Ramachandran R. Vasopressors: Do they have any role in hemorrhagic shock? J Anaesthesiol Clin Pharmacol. 2017 Jan-Mar;33(1):3-8. doi: 10.4103/0970-9185.202185.
- Toung T, Reilly PM, Fuh KC, Ferris R, Bulkley GB. Mesenteric vasoconstriction in response to hemorrhagic shock. Shock. 2000;13(4):267-73. doi: 10.1097/00024382-200004000-00003.
- Sharma RM, Setlur R. Vasopressin in hemorrhagic shock. Anesth Analg. 2005 Sep;101(3):833-834. doi: 10.1213/01.ANE.0000175209.61051.7F.
- Rahbar E, Cardenas JC, Baimukanova G, Usadi B, Bruhn R, Pati S, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015 Apr 12;13:117. doi: 10.1186/s12967-015-0481-5.
- Johansson PI, Stensballe J, Ostrowski SR. Shock induced endotheliopathy (SHINE) in acute critical illness – a unifying pathophysiologic mechanism. Crit Care. 2017 Feb 9;21(1):25. doi: 10.1186/s13054-017-1605-5. Erratum in: Crit Care. 2017 Jul 13;21(1):187. doi: 10.1186/s13054-017-1756-4.
- Bunch CM, Chang E, Moore EE, Moore HB, Kwaan HC, Miller JB, et al. SHock-INduced Endotheliopathy (SHINE): A mechanistic justification for viscoelastography-guided resuscitation of traumatic and non-traumatic shock. Front Physiol. 2023 Feb 27;14:1094845. doi: 10.3389/fphys.2023.1094845.
- Sperry JL, Minei JP, Frankel HL, West MA, Harbrecht BG, Moore EE, et al. Early use of vasopressors after injury: caution before constriction. J Trauma. 2008 Jan;64(1):9-14. doi: 10.1097/TA.0b013e31815dd029.
- Richards JE, Harris T, Dünser MW, Bouzat P, Gauss T. Vasopressors in Trauma: A Never Event? Anesth Analg. 2021 Jul 1;133(1):68-79. doi: 10.1213/ANE.0000000000005552.
- Gómez H, Mesquida J, Hermus L, Polanco P, Kim HK, Zenker S, et al. Physiologic responses to severe hemorrhagic shock and the genesis of cardiovascular collapse: can irreversibility be anticipated? J Surg Res. 2012 Nov;178(1):358-69. doi: 10.1016/j.jss.2011.12.015..
- Chalkias A. Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. Int J Mol Sci. 2023 Dec 15;24(24):17522. doi: 10.3390/ijms242417522.
- Shere-Wolfe RF, Galvagno SM Jr, Grissom TE. Critical care considerations in the management of the trauma patient following initial resuscitation. Scand J Trauma Resusc Emerg Med. 2012 Sep 18;20:68. doi: 10.1186/1757-7241-20-68
- Tsujimoto G, Manger WM, Hoffman BB. Desensitization of beta-adrenergic receptors by pheochromocytoma. Endocrinology. 1984 Apr;114(4):1272-8. doi: 10.1210/endo-114-4-1272.
- Plurad DS, Talving P, Lam L, Inaba K, Green D, Demetriades D. Early vasopressor use in critical injury is associated with mortality independent from volume status. J Trauma. 2011 Sep;71(3):565-70; discussion 570-2. doi: 10.1097/TA.0b013e3182213d52.
- Collier B, Dossett L, Mann M, Cotton B, Guillamondegui O, Diaz J, et al. Vasopressin use is associated with death in acute trauma patients with shock. J Crit Care. 2010 Mar;25(1):173.e9-14. doi: 10.1016/j.jcrc.2009.05.003.
- Advanced Trauma Life Support. 9th ed. Chicago, IL: American College of Surgeons; 2012. American College of Surgeons.
- Sims CA, Holena D, Kim P, Pascual J, Smith B, Martin N, et al. Effect of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusions in Patients With Trauma and Hemorrhagic Shock: A Randomized Clinical Trial. JAMA Surg. 2019 Nov 1;154(11):994-1003. doi: 10.1001/jamasurg.2019.2884.
- Plurad DS, Talving P, Lam L, Inaba K, Green D, Demetriades D. Early vasopressor use in critical injury is associated with mortality independent from volume status. J Trauma. 2011 Sep;71(3):565-70; discussion 570-2. doi: 10.1097/TA.0b013e3182213d52
- Fage N, Asfar P, Radermacher P, Demiselle J. Norepinephrine and Vasopressin in Hemorrhagic Shock: A Focus on Renal Hemodynamics. Int J Mol Sci. 2023 Feb 17;24(4):4103. doi: 10.3390/ijms24044103.
- Zhang B, Dong X, Wang J, Li GK, Li Y, Wan XY. Effect of Early versus Delayed Use of Norepinephrine on Short-Term Outcomes in Patients with Traumatic Hemorrhagic Shock: A Propensity Score Matching Analysis. Risk Manag Healthc Policy. 2023 Jun 22;16:1145-1155. doi: 10.2147/RMHP.S407777.
- Rossaint R, Afshari A, Bouillon B, Cerny V, Cimpoesu D, Curry N, et al. The European guideline on management of major bleeding and coagulopathy following trauma: sixth edition. Crit Care. 2023 Mar 1;27(1):80. doi: 10.1186/s13054-023-04327-7.
- Fox AW, May RE, Mitch WE. Comparison of peptide and nonpeptide receptor-mediated responses in rat tail artery. J Cardiovasc Pharmacol. 1992 Aug;20(2):282-9. doi: 10.1097/00005344-199208000-00014.
- Yamamoto K, Ikeda U, Okada K, Saito T, Shimada K. Arginine vasopressin inhibits nitric oxide synthesis in cytokine-stimulated vascular smooth muscle cells. Hypertens Res. 1997 Sep;20(3):209-16. doi: 10.1291/hypres.20.209.
- Okazaki N, Iguchi N, Evans RG, Hood SG, Bellomo R, May CN, et al. Beneficial Effects of Vasopressin Compared With Norepinephrine on Renal Perfusion, Oxygenation, and Function in Experimental Septic Acute Kidney Injury. Crit Care Med. 2020 Oct;48(10):e951-e958. doi: 10.1097/CCM.0000000000004511.
- Enomoto M, Pan J, Shifrin Y, Belik J. Age dependency of vasopressin pulmonary vasodilatory effect in rats. Pediatr Res. 2014 Feb;75(2):315-21. doi: 10.1038/pr.2013.221.
- Barrett LK, Singer M, Clapp LH. Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit Care Med. 2007 Jan;35(1):33-40. doi: 10.1097/01.CCM.0000251127.45385.CD..
- Russell JA, Walley KR. Vasopressin and its immune effects in septic shock. J Innate Immun. 2010;2(5):446-60. doi: 10.1159/000318531.

Published under the Creative Commons Attribution 4.0 International License
