ENDOVASCULAR MANAGEMENT OF NONVARICEAL GASTROINTESTINAL BLEEDING
Introduction
Non-variceal gastrointestinal bleeding (GIB) is a frequent cause of hospital admission. It can be broadly classified into two groups according to its relationship to the ligament of Treitz: upper and lower GIB. The clinically apparent GIB as visible blood loss may manifest as hematemesis, melena, or hematochezia. Hematemesis refers to vomiting red blood or coffee-ground emesis and suggests bleeding proximal to the ligament of Treitz. Melena is defined as black, tarry stool that occurs several hours after the bleeding event and results from the degradation of blood to hematin or other hemochromes by gut bacteria and can be seen with variable degrees of blood loss, being visible with as little as 50 mL of blood. Hematochezia refers to red or maroon blood in the stool and suggests active bleeding, usually from the lower GIB. It can also be seen in the massive upper GIB, typically associated with hemodynamic instability (1). Types of GIB’s are shown in Table 1.
Table 1. Types of gastrointestinal bleedings
| Type of GIB | Description |
| Upper GIB | GIB originating proximal to the ligament of Treitz |
| Lower GIB | GIB originating distal to the ligament of Treitz |
| Suspected small bowel bleeding | GIB in which no bleeding source is identified after performing both upper and lower endoscopy. |
| Overt GIB | Visible GIB such as hematemesis, hematochezia, or melena. |
| Massive GIB | GIB associated with hemodynamic instability (blood pressure <90 mmHg, tachycardia, symptoms of shock) or bleeding requiring transfusion of more than 4 units of packed red blood cells per 24 hours. |
| Obscure GIB | GIB in which no bleeding source is identified after the entire GI tract has been evaluated with advanced endoscopic and imaging techniques. Can be either overt or occult. |
| Occult GIB | GIB that is not clinically visible (positive fecal occult blood test or iron deficiency anemia when other causes of anemia are excluded). |
GIB – gastrointestinal bleeding; GI – gastrointestinal
Differentiating upper and lower GIB based on the clinical presentation of hematemesis, hematochezia, or melena may be difficult and unreliable. Patients with upper GIB commonly present with hematemesis and/or melena, although those with a brisk upper gastrointestinal (GI) source can present with hematochezia. In 70% of cases, GIBs are located in the upper GI tract (2,3). With an incidence of 50 to 100 per 100 000 population, it is a common pathology with a median patient age of 60–70 years (4). In 70–75 % of cases an upper GIB ceases spontaneously. The mortality rate is between 3 and 14% and for intensive care patients between 42 and 64% (2). In approximately 50% of cases, upper GIB results from an ulcer disease such as a gastric ulcer or duodenal ulcer. Other causes include tumour bleeding, Mallory-Weiss syndrome, erosive gastritis or duodenitis, reflux esophagitis, angiodysplasia and iatrogenic or post-traumatic changes. A special cause of upper GIB is acute bleeding of the peripancreatic vessel branches, which is often the result of pancreatitis, surgery, tumours or trauma.
Lower GIB causes approximately 30% of all GIB, with an incidence of about 20 to 30 per 100 000 population and a median age of 65–80 years, increasing dramatically with age (5). In 80–85% of cases lower GIB ceases spontaneously. Recent studies indicate the mortality between 2 and 5% (2). The most common cause of lower GIB is diverticulitis and less frequent are angiodysplasia, polyps, tumours, proctitis or chronic inflammatory bowel disease (6). A separate group is comprised of hemorrhaging from sources outside the digestive tract, such as the biliary tract, the pancreatic duct, arterioenteric fistula, and visceral artery aneurysms or pseudoaneurysms (7).
Once the likely location of bleeding (upper, lower, or small bowel) has been determined, the list of diagnostic possibilities may be narrowed down based on the patient’s history and risk factors for GIB. Important information includes the type of bleeding (overt, occult, or massive), associated signs and symptoms (e.g., abdominal pain, weight loss), contributing events (e.g., trauma, vomiting, hypotension), and recent procedures (e.g., polypectomy, liver biopsy). Key patient history and risk factors for GI bleeding are listed in Table 2.
Table 2. Key patient history and risk factors for gastrointestinal bleeding
| Angiodysplasia | Advanced age, aortic stenosis, end-stage renal disease, anticoagulant or antiplatelet therapy |
| Aortoenteric fistula | Massive GIB, infectious aortitis, aortic graft, aortic aneurysm, tumor invasion, radiation injury |
| Bowel ischemia | Acute abdominal pain, hypotension, advanced age, embolic disease, chronic renal failure, trauma, high-risk surgery |
| Crohn disease | Risk factors include duration of Crohn’s, perianal disease, left colon involvement, steroid use |
| Delayed postpolypectomy bleeding | Polyp size > 10mm, thick stalk, anticoagulant or antiplatelet therapy |
| Dieulafoy lesion | Antiplatelet therapy, alcohol abuse, NSAID |
| Diverticular bleeding | Painless hematochezia, advanced age, hypertension, anticoagulant therapy, NSAID |
| GI malignancy | Unexplained weight loss, change in bowel habits, anemia |
| Hemobilia | Liver biopsy, cholecystectomy, endoscopic biliary procedures, trauma, tumors, hepatic artery aneurysm |
| Hemosuccus pancreaticus | Pancreatitis (chronic or necrotizing), neoplasm, pseudocyst |
| Mallory-Weiss tear | Vomiting, often related to alcohol abuse |
| NSAID enteropathy or colopathy | NSAID use |
| Postsurgical anastomotic bleeding | Gastric bypass surgery, Billroth II, NSAID use, smoking |
| Peptic ulcer disease | Epigastric pain, nausea, bloating; Helicobacter pylori infection, NSAID, anticoagulant or antiplatelet therapy, stress, Zollinger-Ellison syndrome |
| Varices and portal hypertensive gastropathy | Massive GIB; cirrhosis, portal hypertension, portal vein thrombosis |
GIB – gastrointestinal bleeding; GI – gastrointestinal; NSAID – non-steroidal anti-inflammatory drug
Pretreatment imaging
Scintigraphy is the most sensitive imaging method, with the ability to detect bleeding from 0.1 ml/min (8). However, this technique is not able to define precisely the anatomic source of the bleeding. In addition, scintigraphy is too time-consuming to be used in an emergency setting. Hence, it is mainly used for intermittent bleeding.
Conventional digital subtraction angiography (DSA) is able to detect small bleeding amounts (>0.5 ml/min) (9). Its sensitivity ranges from 63 to 90% for upper and 40 to 86% for lower GI tract (10). The localization of bleeding can be improved by previous placement of metal clips at the bleeding source during endoscopy.
Contrast enhanced computerized tomography (CECT) is the imaging method of choice: it is noninvasive, fast, and more sensitive than digital subtraction angiography (DSA).
Contrast enhanced computerized tomography (CECT) can detect even smaller amounts of bleeding (<0.3 ml/min), and is more sensitive than DSA (10). In addition, compared to DSA, it is able to depict surrounding anatomical structures and to determine not only the place, but also a possible cause of bleeding. Since GIBs are usually intermittent in nature, it is important to scan the patients during the actual bleeding in order to determine the bleeding localization. CECT also displays the complete vascular anatomy and allows planning of subsequent endovascular intervention. Oral contrast should not be administered, because it will mask the intraluminal contrast material, which is a radiological sign of bleeding. Even in hemodynamically unstable patients with acute significant bleeding of obscure localization, CECT should be considered the imaging method of choice due to its non-invasiveness, speed, and sensitivity. Since the CECT is more sensitive than DSA, DSA and embolization should be considered only in cases when bleeding is identified on CECT.
Indications for endovascular treatment
The indication for endovascular treatment is usually based on a multidisciplinary consensus between the gastroenterologist, radiologist, and surgeon. In the event of acute significant gastrointestinal bleeding and after the failure of conservative treatment, endoscopy is the method of choice. Acute significant bleeding is generally considered as bleeding requiring transfusion of at least 4 units of blood within 24h or causing signs of hemodynamic instability and shock (systolic blood pressure <100, tachycardia >100) (11). Endovascular treatment is indicated for patients with significant acute GIB with endoscopically untreatable or unrevealed source of bleeding or with excessive bleeding that obscures the endoscopic view (7). It is recommended to perform CECT before the intervention. In the case of a negative CECT, the probability of detection of the bleeding site in DSA is low. Surgical treatment is generally considered in operable patients, especially those with a bleeding gastroduodenal peptic ulcer (12) or recurrent bleeding from colonic diverticula (13) and after endoscopy and embolization therapy failure.
Contraindications
Contraindications for embolization in significant GIB are only relative. These include general contraindications for iodine-contrast examinations (allergy and renal insufficiency), and those of coagulopathy and residues of barium sulphate contrast agent after the previous examination.
Procedure
Transarterial embolization (TAE) is an endovascular procedure in which embolic agents such as coils, microparticles, or liquid embolic are intentionally introduced in the vessel in order to achieve haemostasis (Figure 1).
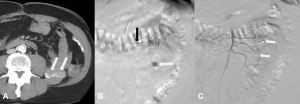
Figure 1. Lower gastrointestinal bleeding (GIB) (hematochezia) with unidentified genesis in a 74-year-old male. A. An axial contrast enhanced computerized tomography (CECT) shows an intraluminal contrast extravasation within a jejunal loop in the left hemiabdomen (white arrows). B. Superselective digital subtraction angiography (DSA) with the tip of the microcatheter in the left colic artery (black arrow) shows active bleeding (white arrow). C. Selective superior mesenteric artery DSA after the embolization shows two coils (white arrows) with complete cessation of the bleeding.
The patient’s preparation before the procedure includes supportive therapy (volume therapy, etc.) and correction of coagulopathy. Bladder catheter insertion is desirable. In patients with GI bleeding, it is necessary to have anaesthesia or intensive care physician support, particularly in unstable patients. During the procedure, blood pressure, heart rate, saturation, and ECG are monitored.
The most common access used for TAE is the common femoral artery, therefore both groins must be shaved before the procedure. The procedure is usually performed under local anesthesia with analgosedation. It is recommended that arterial puncture should be done under ultrasound guidance in order to avoid local complications. Use of spasmolytics (e.g., Buscopan) can be helpful in avoiding motion image artefacts.
The procedure begins with selective angiography to localize the source of bleeding. After verifying the source, a microcatheter is introduced coaxially through the diagnostic catheter. The most commonly used embolic materials are microcoils, PVA (polyvinyl alcohol) microspheres (500-700 um), gelatin foam, and tissue glue (Histoacryl™). Selective intraarterial infusion of vasoconstrictor agent (vasopressin) is rarely used due to the high frequency of rebleeding (>50 %) and occurrence of systemic side effects (14). It could be considered for diffuse mucosal haemorrhage, or lesions inaccessible to a microcatheter.
Due to differences in blood supply of the upper and lower GI tract, the technique of embolization also differs. The upper gastrointestinal tract is characterized by a rich network of collateral supply with a lower risk of ischemia. Before the embolization itself, it is necessary to map all the possible sources of collateral supply, especially in the region of gastroduodenal artery and pancreaticoduodenal arcades. Because of the risk of rebleeding via collaterals, it is necessary to perform embolization proximally and distally from the site of bleeding (the so-called sandwich technique). In the lower gastrointestinal tract, particularly in the colon, there is a higher portion of terminal branches. Therefore, the ischemia risk is higher, and embolization should be as selective as possible (15).
Outcomes
Generally, the morbidity and mortality associated with endovascular intervention for GIB is lower or comparable than for surgical procedure (16,17).Therefore, endovascular therapy is considered the treatment of choice for GIB following failed medical and endoscopic therapy. Predictive factors for recurrent bleeding and mortality are uncorrectable coagulopathy, older age, cirrhosis, oncologic diseases, multiple organ failure, and current corticosteroid treatment (18).
Digital subtraction angiography (DSA) and embolization should be considered only in cases when bleeding is identified on contrast enhanced computerized tomography (CECT).
Complications
In addition to the standard rate of nonspecific complications associated with any angiographic procedure (such as reactions to the contrast agent, renal failure, local complications in the groin, dissection, and vasospasm), the most common and specific complication of GI embolization is ischemia. The risk of ischemia is low in the upper GI tract due to the rich collateral supply. Duodenal stenosis as a result of duodenal ischemia following embolization is rare and reported to be less than 7 %. Patients are at increased risk of ischemia if they have a previous history of surgery or radiotherapy and after embolization with glue or microparticles (11). The overall average complication rate is approximately 9% (19). In the lower GI tract, the most common specific complication is intestinal ischemia. The mild form presented with transient abdominal pain and asymptomatic stenosis occurs in 10 % of patients. Severe ischemic complications requiring surgical treatment (symptomatic ischemic stenosis, intestinal infarction) occur in 2 % (20).
Conclusion
TAE is an effective procedure in the treatment of GIB in patients with a bleeding source not detected on endoscopy or who cannot undergo endoscopy. The clinical success and complications of this approach in upper GIB makes preventive TAE useful in selected patients due to the high risk of rebleeding, also in consideration of the generally limited complications in empirical embolization of the upper gastrointestinal tract. Patient selection should be more prudent in the treatment of lower GIB: due to poor collateral supply lower GI tract is at higher risk of ischemia, hence the treatment should be as selective as possible.
Emergency physicians must recognize the indications for endovascular intervention, prioritize rapid imaging, and facilitate timely multidisciplinary coordination to optimize patient outcomes.
References
- Feldman M, Friedman LS, Brandt LJ, Sleisenger, Fordtran. Gastrointestinal and Liver Disease E-Book. 10th ed. Elsevier Health Sciences NY, USA, 2015; Volume 1, p. 439–451.
- Manning-Dimmitt LL, Dimmitt SG, Wilson GR. Diagnosis of gastrointestinal bleeding in adults. Am Fam Physician. 2005 Apr 1;71(7):1339-46.
- Barnert J, Messmann H. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol 2009;6:637–46. doi: 10.1038/nrgastro.2009.167
- Hreinsson JP, Kalaitzakis E, Gudmundsson S, Bjornsson El. Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol 2013;48:439–47. doi: 10.3109/00365521.2012.763174
- Navuluri R, Kang L, Patel J, Van Ha T. Acute lower gastrointestinal bleeding. Semin Intervent Radiol 2012;29:178–86. doi: 10.1055/s-0032-1326926
- Zhang BL, Chen CX, Li YM. Capsule endoscopy examination identifies different leading causes of obscure gastrointestinal bleeding in patients of different ages. Turk J Gastroenterol 2012;23:220–5. doi: 10.4318/tjg.2012.0338
- Walker TG. Acute gastrointestinal hemorrhage. Tech Vasc Interv Radiol 2009;12:80–91. doi: 10.1053/j.tvir.2009.08.002
- Stunell H, Buckley O, Lyburn ID, McGann G, Farrell M, Torreggiani WC. The role of computerized tomography in the evaluation of gastrointestinal bleeding following negative or failed endoscopy: a review of current status [review]. J Postgrad Med 2008;54(2):126–34. doi: 10.4103/0022-3859.40779
- Baum ST. Arteriographic diagnosis and treatment of gastrointestinal bleeding. In: Baum ST, Pentecost MJ editors. Abram’s angiography interventional radiology, 2nd edn. Lippincott Williams & Wilkins, Philadelphia; 2006, p. 488.
- Laing CJ, Tobias T, Rosenblum DI, Banker WL, Tseng L, Tamarkin SW. Acute gastrointestinal bleeding: emerging role of multidetector CT angiography and review of current imaging techniques. Radiographics 2007;27:1055–70. doi: 10.1148/rg.274065095
- Loffroy R, Guiu B. Role of transcatheter arterial embolization for massive bleeding from gastroduodenal ulcers. World J Gastroenterol 2009;15:5889–97. doi: 10.3748/wjg.15.5889
- Defreyne L, De Schrijver I, Decruyenaere J, Van Maele G, Ceelen W, De Looze D et al. Therapeutic decision-making in endoscopically unmanageable nonvariceal upper gastrointestinal hemorrhage. Cardiovasc Intervent Radiol 2008;31:897– 905. doi: 10.1007/s00270-008-9320-x
- Lee J, Costantini TW, Coimbra R. Acute lower GI bleeding for the acute care surgeon: current diagnosis and management. Scand J Surg 2009;98:135– 42. doi: 10.1177/145749690909800302
- Gomes AS, Lois JF, McCoy RD. Angiographic treatment of gastrointestinal hemorrhage: comparison of vasopressin infusion and embolization. AJR Am J Roentgenol 1986;146: 1031–7. doi: 10.2214/ajr.146.5.1031
- Valek V, Husty J. Quality improvement guidelines for transcatheter embolization for acute gastrointestinal nonvariceal hemorrhage. Cardiovasc Intervent Radiol. 2013;36:608-12. doi: 10.1007/s00270-012-0462-5
- Eriksson LG, Ljungdahl M, Sundbom M, Nyman R. Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol 2008;19:1413–8. doi: 10.1016/j.jvir.2008.06.019
- Langner I, Langner S, Partecke LI, Glitsch A, Kraft M, Bernstorff W et al. Acute upper gastrointestinal hemorrhage: is a radiological interventional approach an alternative to emergency surgery? Emerg Radiol 2008;15:413–9. doi: 10.1007/s10140-008-0736-z
- Schenker MP, Duszak R Jr, Soulen MC, Smith KP, Baum RA, Cope C et al. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol 2001;12:1263–71. doi: 10.1016/s1051-0443(07)61549-8
- Loffroy R, Rao P, Ota S, De Lin M, Kwak BK, Geschwind JF. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol 2010;33:1088–100. doi: 10.1007/s00270-010-9829-7
- Weldon DT, Burke SJ, Sun S, Mimura H, Golzarian J. Interventional management of lower gastrointestinal bleeding [review]. Eur Radiol 2008;18:857–67. doi: 10.1007/s00330-007-0844-2

Published under the Creative Commons Attribution 4.0 International License
